Fluoride: a natural substance that prevents tooth decay
False claims about fluoride use fear-based messaging to spread disinformation.
It is exhausting when prominent “science personalities” spread harmful misinformation that erodes literacy and public health. Unfortunately, fluoride, which has been used for over 75 years to prevent tooth decay, is a common victim of this.
Fluoride is a naturally-occurring component of minerals found in water and foods.
It is released during natural geologic processes by rocks into the soil, water, and air. Fluoride is not found in isolation, but rather, as a constituent of fluoride-containing compounds (“fluoride” is not an element, but an ion, with formula F-). Elemental fluorine is F2 and at standard conditions is in gaseous form.
Fluoride ion (F-) can be found in both inorganic and organic compounds (in chemistry, organic means something VERY different than how it is used to refer to commercial consumer products).
Inorganic fluoride compounds include minerals like fluorite (CaF ), fluoroapatite, sometimes called calcium fluorophosphate (Ca5(PO4)3F), and cryolite (Na3AlF6). Fluoride can also be found in inorganic salts like sodium fluoride (NaF), potassium fluoride (KF), calcium fluoride (CaF2), and others.
While the public thinks of “salt” as table salt (sodium chloride, NaCl), salt in chemistry means any ionic compound formed after an acid:base neutralization reaction and generally contain a metal cation (positive ion) and a halogen anion (negative ion).
Organic molecules are those that are typically carbon-containing. Some classes of organic compounds containing fluoride include fluoroacetates and fluoroalkanes. These are typically produced by plants to deter predators (for example, there are many fluoroacetates that are toxic to various animals upon ingestion).
In the atmosphere, you can also find hydrogen fluoride gas (HF), and fluoride can react with other chemicals and elements in the environment. Fluoride ion is one of the most reactive ions, so fluoride is ubiquitous on the earth.
Fluoride is found most often in minerals and rocks and during erosion and weathering processes, fluoride ions are released from those sediments where it enters the soil, groundwater, etc. As a result, fluoride is naturally found in many plants (including those we consume), in groundwater, in surface water, and more.
Fluoride is naturally present in various environmental sources:
Water: Fluoride is naturally found in many water sources, especially groundwater. The concentration of fluoride in water can vary depending on geological factors and the presence of fluoride-containing minerals in the soil and rocks.
Soil: Fluoride-containing minerals such as fluorite and fluoroapatite are commonly found in soil.
Air: Fluoride compounds can be released into the air through natural processes like volcanic eruptions and dust storms.
Plants: Certain plants accumulate fluoride from the soil through their roots. Tea leaves, for example, are known to contain relatively high levels of fluoride. On average, fluoride in tea leaves ranges from approximately 1 to 10 milligrams of fluoride per kilogram of dry weight (1 to 10 parts per million, ppm), but can often be up to 20 ppm or greater, depending where the tea was grown.
Other foods: Fluoride can also be naturally found in other food sources like seafood and beverages that contain water.
Our teeth structure can be impacted by demineralization, which can lead to tooth decay and dental disease.
Our teeth are complex living tissues which are susceptible to wear and tear from the environment. The hard exterior of the tooth, the enamel, can demineralize and weaken as a result of external factors.
Acid Production by Bacteria: Your oral microbiome metabolize sugars and carbohydrates from food you consume. During this digestive process, bacteria produce acids as byproducts, which can react with tooth enamel and deteriorate it.
Drop in pH: In addition, those acids produced by bacteria also reduce the pH level in the mouth, which can further acidify the environment and weaken the structure of tooth enamel.
Enamel demineralization: The acidic environment dissolves the mineral crystals (primarily hydroxyapatite) that make up the enamel, which releases calcium and phosphate ions from the enamel, making it weaker and more susceptible to decay.
Dental Caries: If this process occurs repeatedly without repair, areas of enamel may develop holes, forming cavities (dental caries). If left untreated, caries can progress deeper into the tooth, affecting the dentin (the layer beneath the enamel) and potentially leading to more extensive decay and tooth damage.
Fluoride exposure improves tooth enamel strength by shifting hydroxyapatite to fluoroapatite.
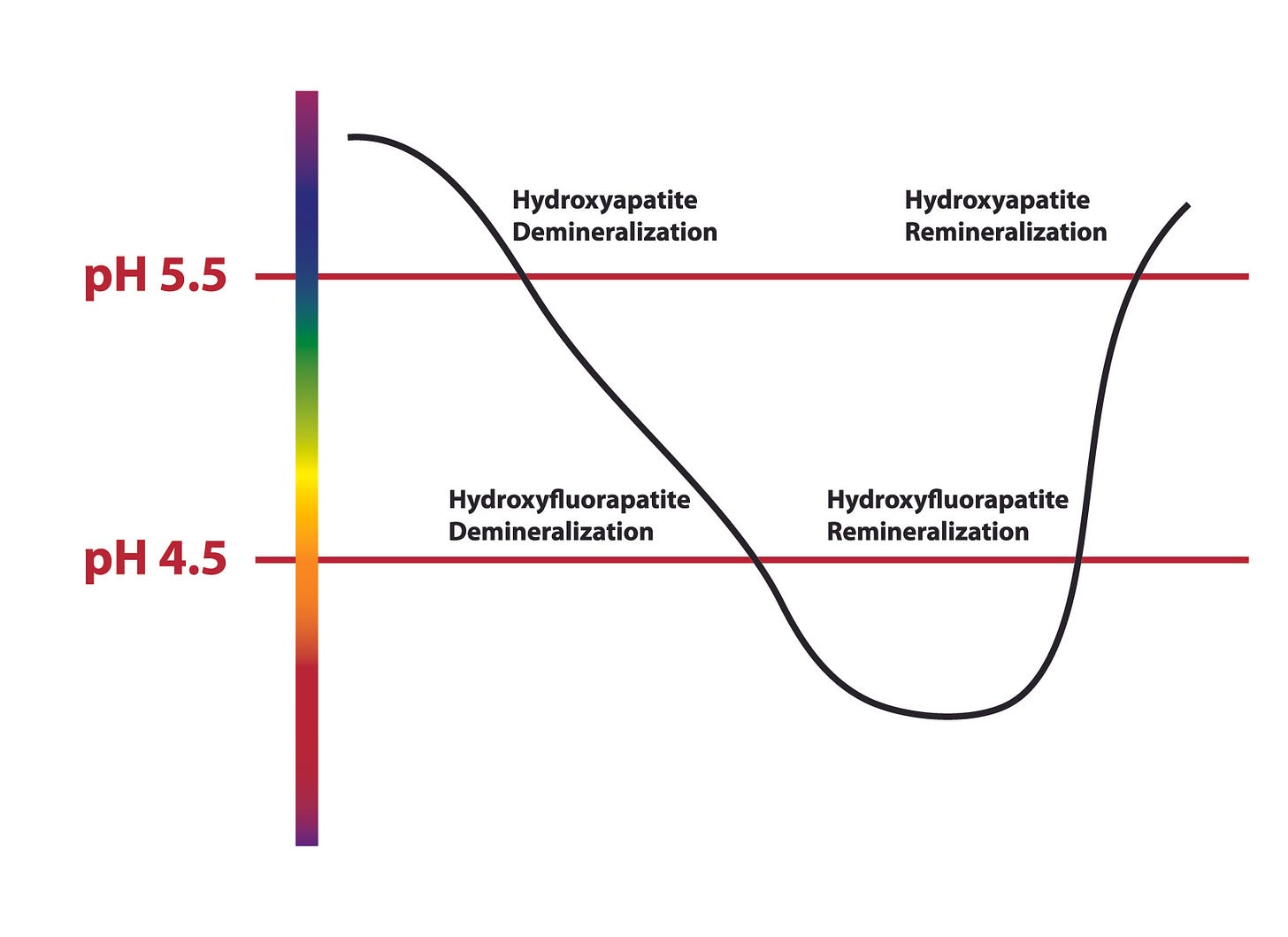
As I mentioned, our normal tooth enamel is composed of a mineral network, primarily (70-80%) made up of hydroxyapatite (Ca₁₀(PO₄)₆(OH)₂). However, hydroxyapatite can be dissolved in an environment of pH 5.5, which can occur as the oral cavity is acidified.
However, in the presence of fluoride ions, the hydroxide ions in hydroxyapatite (OH-) can be replaced by fluoride (remember when I said fluoride is very reactive? This is because of a feature called electronegativity - fluoride is very strong and can displace other ions). When fluoride replaces the hydroxide, you form hydroxyfluoroapatite, or fluoroapatite for short. Fluorapatite is a more stable crystalline structure than hydroxyapatite.
This is where the science gets really cool: fluoroapatite doesn’t start to dissolve until the pH drops to 4.5 (this is an order of 10-fold, meaning 10 times more acidic). What that means is, your teeth that have enamel made of fluoroapatite are much stronger and can withstand a more acidic environment than if they only contain hydroxyapatite.
Fluoride helps strengthen tooth enamel which helps protect teeth and prevent decay caused by bacteria. Fluoride is primarily effective topically (meaning through direct contact) with the teeth. Raising the levels of fluoride in the mouth makes it more available to be incorporated into tooth structure, which means that the natural hydroxyapatite of tooth enamel is altered to fluoroapatite.
Fluoride enhances enamel remineralization and deposition of calcium and phosphate ions into tooth enamel through the formation of the fluorapatite, which is more resistant to acid attack than the original hydroxyapatite structure of enamel. As a result of the formation of fluoroapatite, the solubility of enamel in acidic environments is reduced, making your teeth more sturdy even in harsher environments. Fluoride in your saliva promote the uptake of calcium and phosphate ions from saliva into the tooth structure, which helps repair weakened areas of enamel in real-time. As an added bonus, fluoride ions interfere with the metabolism of certain oral bacteria species, which reduces their acid-producing capabilities. This means that fluoride also helps stabilize the pH in the mouth, to reduce the potential erosive effects of acids.
Fluoridated water prevents tooth decay.
Fluoride is added to community drinking water to increase the concentration to a recommended level through a process called fluoridation. Fluoride is also found in dental health products, beverages, food, some dietary supplements, and medications.
Fluoride is added to water in the form of sodium fluoride (NaF), fluorosilicic acid (H2SiF6), or sodium fluorosilicate (Na2SiF6). Community water fluoridation has been used in the United States for over 75 years, and it has been credited with massive improvement in dental health outcomes. On average, young children in areas with fluoridated water have two fewer decayed teeth than children of a similar age in areas without fluoridated water.
The federal recommendation of fluoride levels in the United States is 0.7 parts per million (ppm). As of a recent estimate in 2018, 63% of the US population receives fluoridated water at a community level. The resultant declines of cavities in the U.S. since the widespread implementation of fluoridation have contributed to its designation per the CDC as one of the top 10 public health achievements of the 20th century. The vast majority of medical organizations recommend the use of water fluoridation, including the American Academy of Pediatrics, the World Health Organization, the American Dental Association, and the U.S. Public Health Service.
Recent studies have added to the evidence that fluoridation protects against tooth decay due to sugar consumption among children and that cessation of community water fluoridation was associated with greater tooth decay for children compared to those in communities that continued fluoridation.
For example, Buffalo, NY removed fluoride from the public water supply in 2015, and since then, increased cavities and other dental problems, particularly among children, have occurred. Studies examining communities that have halted fluoridation have shown increases in cavities ranging from 25% to 50%.
In areas of England, a country where community water fluoridation is much less common, children were found to have less tooth decay and fewer hospital admissions for tooth extractions if they lived in an area with community water fluoridation. Systematic reviews have demonstrated similar effectiveness of fluoridation among adults, resulting in a roughly 25 percent reduction in tooth decay.
Fluoride is NOT toxic at the levels we are exposed to.
I get it, if you listened to social media influencers, you’d hear all sorts of fear-mongering. Fluoride has been demonized since it was implemented as a tool to prevent tooth decay. And people who claim it’s a toxin forget that ANYTHING can be toxic at a certain exposure (yes, Huberman, I am once again looking at you).
It would be extremely difficult, if not impossible, to experience acute toxicity from drinking fluoridated water based on the levels implemented in the US (0.7 parts per million). The conservative threshold for fluoride toxicity is based on impacts to skeletal bones. That level is 4 milligrams/ kg of body weight/day. Which means a kid weighing 10 kg (22 lbs) would need to drink 57 LITERS of fluoridated water to hit that. You’d experience toxicity from the water itself well before the fluoride (yes, water can be toxic). For claims relating to neurotoxicity, that level is even higher. For other claims such as impacting thyroid function, it’s also higher (and unsubstantiated).
Note: It is possible to have high exposures if one were to consume toothpaste in large quantities, but that is an entirely different situation altogether, and toothpaste is not meant for consumption, especially in large quantities.
Fluoride doesn’t damage teeth, cause cancer, or reduce IQ.
Dental Fluorosis:
Fluoride exposures at higher doses can cause fluorosis, which are aesthetic changes to the tooth enamel, resulting in white or brownish spots. In fact, this is how fluoride was discovered to improve dental health! In the early 20th century, it was noted that individuals residing in certain areas with naturally high fluoride levels in drinking water exhibited a lower prevalence of dental caries (cavities), but they also had tooth mottling and discoloration. Fluorosis does not have any negative effect on function, is not painful, and is typically difficult to see except by a trained professional. Severe cases are extremely rare. In fact, fluorosis of dental enamel may even make teeth more resistant to decay.
Fluoride and Cancer:
Fluoride absolutely does NOT cause cancer. There is over 50 years of research to support this. Extensive research spanning more than half a century has found no evidence of an association between cancers of any kind and fluoride exposure in humans. More than that, studies specifically assessing potential linkages between bone cancers and fluoride exposure have failed to find any association.
Fluoride and IQ:
This claim was borne from misinterpretation of a selection of cherry-picked studies that investigated fluoride levels and exposures at least two to three times greater than levels present in the United States. There were many confounding factors in the study (such as concurrently high arsenic exposure, asbestos exposure, coal burning practices, etc.), leading experts to advise caution when interpreting the data. There is no robust body of evidence to suggest there is any relationship between fluoride exposure and IQ level. As a result, all dental expert agencies recommend fluoride use in children and pregnant people.
Hydroxyapatite is not superior to fluoride for tooth health.
Hydroxyapatite (HAP) is a calcium phosphate mineral that is the main inorganic component of human hard tissues, like teeth and bones. Many people on social media tout using HAP toothpaste over fluoride toothpaste, since it’s the “natural” component of teeth.
However, if you recall from above, fluoride actually improves the strength of tooth enamel as it reduces the solubility of enamel minerals. Fluoride shifts the pH at which minerals are lost from 5.5 to 4.5, so teeth can tolerate more acid before minerals are lost. As such, fluoride is actually superior to HAP when it comes to dental strength.
In addition, HAP does not have the same antimicrobial impacts that fluoride does. Fluoride disrupts bacterial cell membranes and has a bacteriostatic effect, which can further reduce the growth and metabolism of acid-producing bacteria in the mouth in addition to the additional resistance of oral acids.
Moreover, research on HAP is not as robust as for fluoride, which has over 75 years of data. While the broad consensus is that HAP is also safe for use, HAP is less effective, and much more costly. HAP is about ten times as expensive as fluoride and requires a higher concentration to achieve efficacy (at least 10% v. 0.22%, respectively), and is eroded at a higher pH (less acidic environment) that fluoroapatite.
Fluoride-containing toothpastes are much lower cost than HAP-containing counterparts. In addition, water fluoridation is extremely cost-effective. The ADA estimates that every dollar invested per person in fluoridation prevents roughly $38 in dental treatment costs. If you want to check whether your local community’s water is fluoridated, you can do so using this link on the CDC website, and look at water by county.
Fluoride is a critical component of preventive dental health.
Preventive healthcare ensures we can avoid medical issues before they arise. Fluoride does that for our teeth. The data demonstrate that fluoride is indeed safe and effective at protecting our dental health. Fluoride is one of the easiest modifiable factors that reduce the risk of dental caries.
If you hear people spreading misinformation, remember that you are being misled by people who aren’t giving you the truth or the reality. They are scaring you out of things that have been demonstrated to be beneficial over decades. Please, try not to fall prey to fear-mongering and disinformation. It harms you, it harms your kids, and it harms our society. Elevate real information instead.
As always, thanks for joining in the fight for science!
Thank you for supporting evidence-based science communication. With outbreaks of preventable diseases, refusal of evidence-based medical interventions, roadblocks to scientific progress that improve food and crop sustainability, it’s needed now more than ever.
Your local immunologist,
Andrea






Thank you for clarifying an issue that has caused plenty of confusion, thanks to disinformation. I was raised with fluoride (in water, in toothpaste, in dental cleaning products), and I have never had a cavity. (I also credit excellent oral hygiene and outstanding orthodontics.) This was a vastly different outcome than what my parents’ generation and those before them experienced. It makes me so sad to talk with parents who have leapt into this rabbit hole, and are now essentially ruining their kids’ teeth. The death of expertise (replaced by individuals who claim to do their own research - with no understanding of research methodology and no training) is causing so much damage.
As a dentist who graduated from dental school in Buffalo, New York in 1986, I can attest to the effectiveness of fluoridation. It was extremely difficult to find children to work on to achieve our requirements because of how effective fluoridation was at preventing tooth decay. I don’t feel comforted by the fact that young dentists in Buffalo probably do not have that challenge anymore.