Misinformation & public outcry harms health & safety: the paraben case study
Unfounded fears about safe antimicrobial preservatives illustrate the snowball effect of scientific falsehoods.
I’ve talked a lot about broad impacts of science misinformation recently, especially since the recent sham “Expert Panel” in our Congress (read here). I have more to say about the lies spread by those panelists, but it’s important to note that is not a unique situation.
Nearly all anti-science rhetoric comes from poorly interpreted studies, usually mischaracterized by someone who lacks the skills to analyze data properly. When they are grabbed ahold of by media outlets, celebrities, influencers, and politicians, it gives those false claims free reign to spread virulently.
Misinformation spreads further and faster because only 28% of Americans have civic science literacy: the ability to find, understand and use science information to participate in discussions or policy decisions that involve science and technology.
That 28% applies to lawmakers too, which is why policies and laws emerge that are not aligned with the body of evidence on a topic (read more here).
Today, I want to talk about another example that underscores the importance of stopping the spread of science misinformation, even if it seems relatively harmless. This example: parabens.
I presented a version of the paraben story at a recent cosmetics e-conference. Watch the entire conference (or just jump around to the topics that pique your interest!)
Parabens are incredibly safe preservatives that protect many products from harmful microbial contamination.
I’m sure you’ve seen a skincare or beauty product with a label that says “free from parabens” or “no parabens” or similar, right? And you may have thought to yourself: "wait, do I need to be avoiding parabens?”
Well, the answer is no, but you can thank social media and the misinformation landscape. In reality, there is no reason to fear parabens, but public outcry, chemophobia, and low science literacy has created a marketplace where companies have been forced to change chemical formulations because of false claims. That’s not a good thing, but we will get there.
Parabens are a class of chemicals that are esters of 4-hydroxybenzoic acid. That means the hydroxide group is removed and replaced with an ester linkage (-O-) with a functional group, like a methyl-, ethyl-, and so on. Just like with all classes of chemicals, they are each different, and those functional groups are reflected in their names.
Parabens have been used as preservatives since the 1920s without ill consequences. They were found to efficiently inhibit the growth of bacteria, molds, and yeasts in various substances, and as they are are very stable, low in cost, and work on a broad array of microorganisms, they’ve been widely used for 100 years in cosmetics, skincare products, and even medical products.
However, in the last several decades and with the rise of social media, the appeal to nature fallacy, and the “clean beauty” industry placed undue scrutiny on synthetic chemicals, like parabens.
False claims and fear about parabens accelerated with a poorly conducted study in 2004 that was plastered across media outlets.
This paper in the Journal of Applied Toxicology, should have been rejected by peer-review. But it coincided with other anti-science sentiment, and these keywords: cancer, hormones, chemicals - got it a lot of attention.
This study took existing breast tumor biopsies in cold storage and tried to fabricate a problem. Basically, these authors went on a fishing expedition.
They digested the tumor biopsies and used sensitive analytical chemistry methods to assess levels of different parabens. Oh, and there were (20) samples, total. Not an impressive sample size, if you’re wondering.
Before we get into the data itself, the study has several glaring issues.
First: there was no control group. There were no healthy breast tissue samples to normalize values to. The authors suggest parabens might be related to breast cancer, yet don’t have what levels in healthy tissue are to compare.
Second: there is no biological relevance. These data can’t make any conclusions aside from the fact that some paraben may or may not exist in a frozen sample. There is no causal relationship that is being studied, no relationship between stage of cancer or progression or cancer. These samples aren’t taken over a timecourse to assess accumulation or even controlled for types of paraben exposures.
Third: the data itself. They are reporting miniscule levels of different parabens: the mean values across these 20 samples range from 0 ng/g to 12.8 ng/g. For reference, a nanogram per gram is the same functionally as a part per billion: 1 in 1,000,000,000.
For context: 1 part per billion = 1 second in 31.7 years of time
It gets better: the blank values suggest the samples and the instrumentation had contamination issues.
The blank values should represent background measurements: essentially accounting for noise or sample handling contamination issues. These are very critical when dealing with extremely sensitive instrumentation, because a tiny bit of contamination can skew the data substantially.
Which is what it looks like here. These blank values that are reported are frequently higher than the “measured” paraben levels in tumor samples. This suggests that the parabens that were detected in those samples might not actually be from the samples, but might have come from their poor sample handling methods. As such, none of these data can actually be trusted.
The authors also decided to just add “total parabens” together to make the values look bigger, which is wholly inappropriate. That’s like saying “mushrooms” are harmful: when we know every mushroom type is different, and some can be harmful at certain doses, whereas others are perfectly safe, even if we consume them in copious amounts.
Fourth: this is association, not causation. Even if we assume these miniscule levels of parabens are real and were present in breast tumor samples, the detection says nothing. It doesn’t say whether parabens were involved in the cancer in any way. It doesn’t suggest a possible mechanism of how parabens might be implicated in cancer, either.
There are other issues too, including a lack of dose-response and source assessment of paraben exposure, but I think you get the point.
But what this did was fuel the misinformation fire. These levels were taken out of context, wildly exaggerated, and then used to make unfounded claims about parabens being linked to cancer.
What added to this fear were statements that parabens are hormone disruptors. Specifically, that parabens are estrogenic.
Parabens are not estrogenic in a way that would actually have biological relevance.
When we say something is estrogenic, it means that it mimics estrogens in a way that tricks the body into doing something that estrogens would normally trigger. Estrogens bind to and activate estrogen receptor and can lead to a variety of cellular responses, including cell growth, differentiation, and survival.
But when we say parabens have weak estrogenic activity, I mean weak. When we look at the ability of different parabens to bind to estrogen receptor and lead to a biological response, their binding affinity is THOUSANDS of times weaker than actual estrogens.
Butylparaben and propylparaben have 10,000-fold lower binding affinity to estrogen receptor than estradiol, and methylparaben and ethylparaben have 100,000-fold lower binding affinity to estrogen receptor than estradiol. This weak affinity is not at a level that we would anticipate these chemicals to actually be able to activate estrogen signaling.
Unfortunately, once the phrases “hormone disruptor” and “synthetic chemicals” are out in the ether, it is incredibly hard to combat it with the complex nuance that these conversations warrant.
Over 300 studies demonstrate that parabens in consumer products are safe and effective.
Yes, there are decades of data that specifically examine the safety, toxicology, and potential effects of parabens. These include toxicological studies, epidemiological research, animal studies, clinical trials, and regulatory reviews. These have all concluded that parabens, used as anti-microbials, have no link to health effects.
There is no link between parabens and breast cancer - or other cancers.
In fact, regulatory agencies have even created an acceptable daily intake (ADI) for parabens: meaning the level you can ingest, daily, for your entire life, without having any potential health risks. That ADI level is 10 mg/kg body weight/day.
That means that a person weighing 70 kg (154 lbs) could EAT 700 mg (0.7 grams) of parabens daily for their entire life and never be concerned.
Topical use of parabens leads to between 1% and 10% of the total paraben content potentially being absorbed by the skin. This is microgram quantities (1 gram = 1,000 milligrams = 1,000,000 micrograms).
For reference, paraben concentrations in skincare products are between 0.01% and 0.3%, which means 0.01 g to 0.3 g per 100 g of product.
So if you applied 100 g of product (3.6 ounces) - which people are not doing because that is a LOT of a cosmetic product - but *maybe* 0.03 g would be absorbed. You’d literally need to soak in a tub of these products every day, for the rest of your life, to even have a slight chance of risk.
Animal studies found that reproductive issues after paraben exposure appeared only when doses - ingested - were above 1,000 mg/kg body weight/day for long periods of time. These levels are 100-times higher than the ADI for humans - and thousands of times greater than what humans are *actually* exposed to in topical products.
On top of that, parabens are rapidly metabolized into 4-hydroxybenzoic acid and excreted in urine as waste, so parabens are not accumulating in our bodies.
This is why global safety, toxicology, and regulatory agencies all conclude parabens in cosmetics are safe for use.
These include the US Food and Drug Administration (FDA), European Commission's Scientific Committee on Consumer Safety (SCCS), The World Health Organization (WHO), International Programme on Chemical Safety (IPCS), Europeans Medicines Agency, and the Cosmetic Ingredient Review (CIR).
But decades of data and reassurance from safety and chemistry experts didn’t stop media misinformation.
Even today, 20 years after this terrible study, countless articles undermine the safety of parabens.
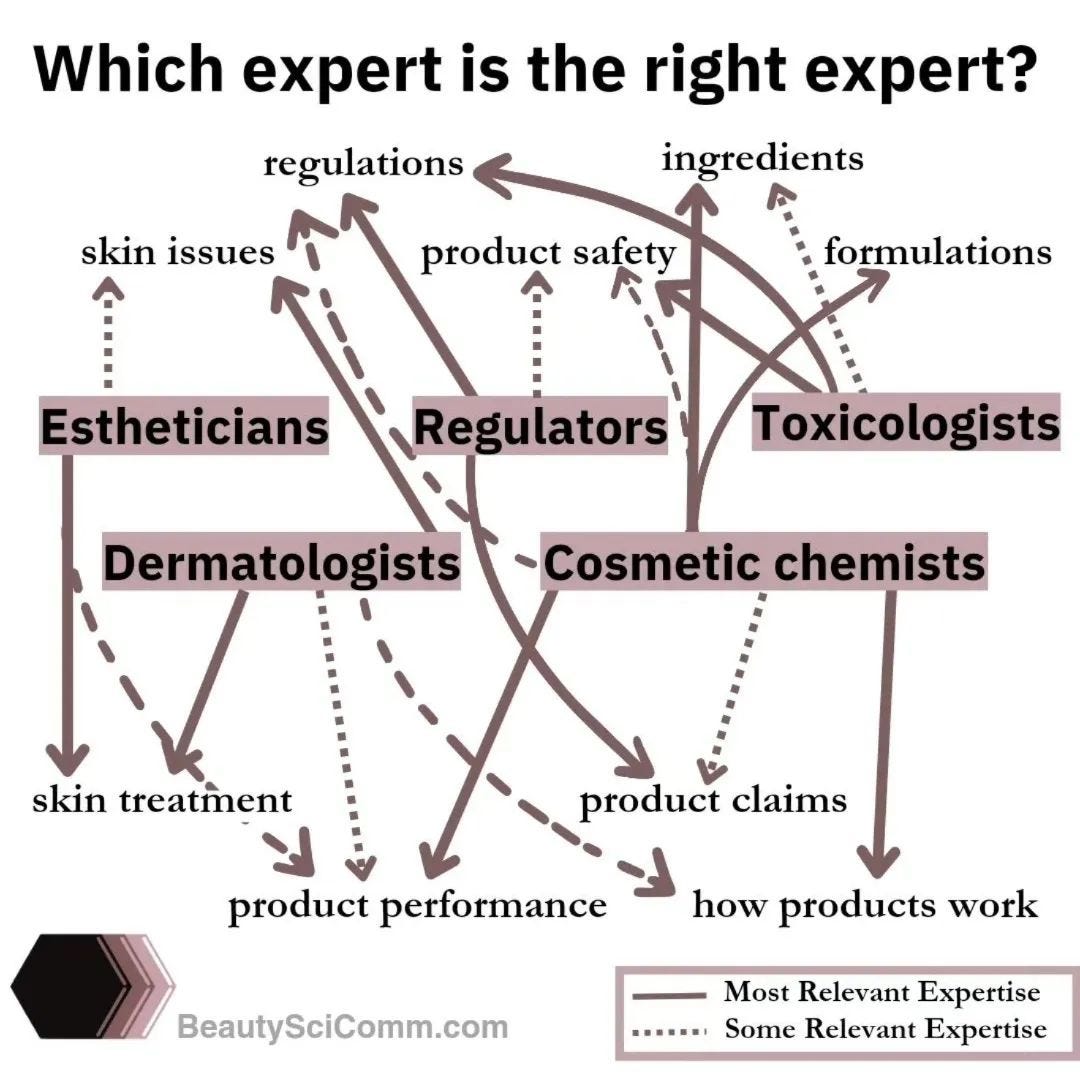
Misinformation even comes from some dermatologists, who, worth noting, are not the experts in chemistry, formulations, skin product safety, etc. (Dr. Michelle Wong and Jen Novakovich have a wonderful chart on who the right expert is on cosmetic topics)
You can imagine what happened.
Public outcry forced companies to reformulate products with alternative preservatives.
This is not a good thing. Parabens, as I mentioned, have broad-spectrum antimicrobial activity, are stable, and inexpensive. Alternatives that companies were left with did not check all of those boxes, which means that inferior and/or more expensive products were the result.
Paraben replacements include:
Phenoxyethanol
less effective against fungi: not full-spectrum.
less stable across formulation ranges: needs to be augmented.
needs higher concentrations for similar effectiveness.
more expensive AND needs to be combined with other antimicrobials, stabilizers, used at higher concentration: increases total cost of materials, manufacture, and consumer price.
Methylisothiazolinone (MIT)
less effective against fungi: not full-spectrum.
less expensive but needs to be combined with other antimicrobials in order to have broad-spectrum coverage.
less safe: MIT has high allergenic potential compared to parabens. Allergies to MIT affect roughly 10% of the population versus 1% of the population with allergies to parabens (which is quite low allergenicity, in case you were wondering).
As a result of this shift to MIT use dermatologic allergies skyrocketed, particularly when MIT was included in leave-on products like cosmetics.
Some companies just opted out of preservatives outright.
Preservatives are used for a reason. The “clean” beauty space drove an abundance of preservative-free products on the market. These are plagued with pathogenic microbe contamination issues. This means skin infections, eye infections, and systemic illness risks for people using these products.
A non-exhaustive list of contamination-associated recalls includes:
Yes To Baby Carrot Wipes (2013) had microbial contamination with Burkholderia cepacia, a bacteria that can cause serious respiratory infections in immunocompromised individuals.
The Honest Company (2017) Organic Baby Powder had a recall due to microbial contamination and risk of skin or eye infections in preservative-free baby powders.
Burt‘s Bees Baby wipes were recalled on several occasions because of Pseudomonas aeruginosa, Pseudomonas fluorescens, and Enterobacter gergoviae contamination.
Beautycounter Baby Soothing Oil had Staphylococcus aureus contamination (S. aureus can cause skin infections, impetigo, and more serious infections like cellulitis or sepsis)
Babyganics, a brand that markets as “free of harmful chemicals,” had bacterial contamination with preservative-free baby wipes and lotions.
Numerous indie and small beauty company products had Staphylococcus epidermidis and Pseudomonas aeruginosa contamination after routine inspection in face creams, lotions, soaps, and more. Recalls accelerated starting in 2019 by FDA, MHRA, and the European Commission.
Regulatory agencies have issued warnings about dangers of under-preserving cosmetics. Preservatives are necessary for product safety especially for products frequently exposed to warmth, moisture, air, or contact with the skin (like products stored and used in… humid bathrooms?)
But paraben fear doesn’t just impact cosmetics.
Aside from the fact that everyone uses some skincare or cosmetic product (lotions, face wash, sunscreens, shampoo/conditioner), disinformation and forced reformulation extends to medical products. Yes, parabens are also important preservatives there, too.
Parabens are used in an array of medical products including:
Topical creams like antifungal creams, corticosteroids, and antibiotic ointments.
Ophthalmic products like eye drops and contact lens solutions.
Medications like injectable anesthetics and some vaccines.
These also have had contamination issues as a result of reformulating with alternative preservatives or no preservatives. Contamination issues with ophthalmic products can lead to serious eye infections, keratitis, and blindness. Contamination among topical and injectable medications also increase the occurrence of systemic infections including cellulitis, sepsis, and other serious infections.
The snowball effect of science disinformation has widespread harms.
Science misinformation is a public health issue. The paraben case is one of many that illustrate the broad impacts when falsehoods are able to spread, unchecked.
This ripple chart I created aims to visualize the amplification effect of false health and science claims. Please feel free to share it if it if you find it useful!
But it goes to the crux of the issue: the broad harms of anti-science rhetoric, clickbait, and legislation that doesn’t align with the body of evidence. When falsehoods and myths are legitimized, it causes harm to all of us.
Thank you for supporting evidence-based science communication. With outbreaks of preventable diseases, refusal of evidence-based medical interventions, propagation of pseudoscience by prominent public “personalities”, it’s needed now more than ever.
Stay skeptical,
Andrea
“ImmunoLogic” is written by Dr. Andrea Love, PhD - immunologist and microbiologist. She works full-time in life sciences biotech and has had a lifelong passion for closing the science literacy gap and combating pseudoscience and health misinformation as far back as her childhood. This newsletter and her science communication on her social media pages are born from that passion. Follow on Instagram, Threads, Twitter, and Facebook, or support the newsletter by subscribing below:











Great case study. It gets even more confusing out in the media-verse because terms are used interchangeably - I suspect on purpose. Phenol is utterly different from benzoic acid - but they get conflated and then folks who have no science literacy are off and running.
It is frustrating in the extreme. My wife is a retired Pediatric NP and smart - but her first response when I sent her your article was: "I just read the other day that parabens can cause arrhythmias."
It took me a while to track down a study that suggested that butylparaben might have "pronounced effects" on the cardiovascular system of Daphnia magna - a plankton.
I tried to explain parabens. She just looked at me and said - "I love you, but I got nothin'." For her, it was so much birdsong.
Oy.