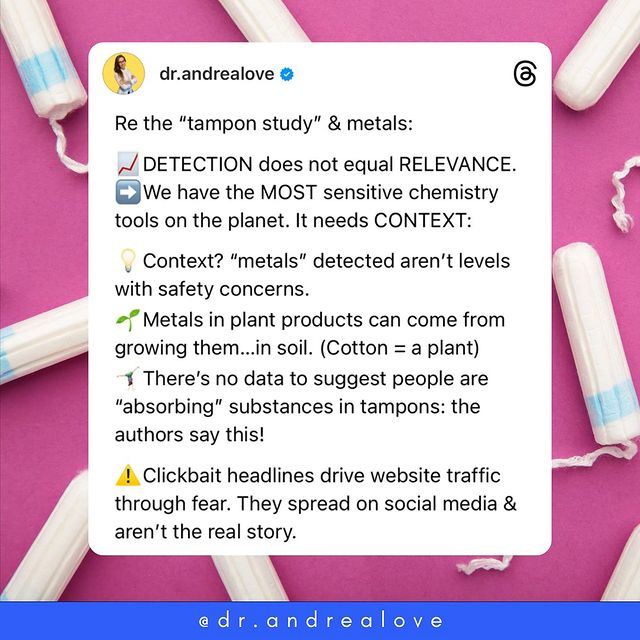
Harmful Levels of Lead in Tampons?! Not so fast...
Once again, clickbait headlines misinterpret weak data and spread fear
Another day, another wildly mischaracterized paper going viral online, eroding science literacy, exacerbating chemophobia, and making our jobs as scientists harder.
If people didn’t scrape PubMed or academic websites for press releases looking for [scary buzzwords], this study wouldn’t have even been a footnote. The research model is not relevant to humans, the methodology has major limitations, there were no biomedical scientists involved in the study, and sample sizes and variability in data undermine any conclusions that can be made.
But since everyone is now terrified of tampons because headlines and unqualified influencers are posting about the TOXIC metals in them, let’s discuss.
The bottom line? No, you do not need to throw out your tampons.
I am going to dissect the study and the context for the data they report. I hope this helps you understand WHY relevant expertise is critical: both to conduct studies in a given field and when media outlets use sources to avoid misinterpreting science.
Lead levels detected are not levels that are of concern for health.
Before I pick apart the actual methods, let’s talk about the reported levels. While there is a list of metalloids, I’m going to focus on lead because that’s garnered the most fear-laden headlines. The conclusions I make are applicable to all.
The study reports a geometric mean of 120 nanograms of lead per gram of tampon material. 120 ng/g is essentially the same as 120 parts per billion.
A part per billion is equivalent to 1 second in 31.7 years of time.
Think about the perspective of that for a minute.
They report a maximum level of 468 ng/g, which means one of the 24 sample samples reported that value.
I weighed a tampon at home (as did Dr. Jen Gunter in her piece on this, which I encourage you to read), and it was 2 grams. Based on the mean of 120 ppb and the maximum of 468 ppb, you’re looking at 240 nanograms or 936 nanograms of lead per tampon.
These are miniscule quantities. 240 nanograms is 0.00000024 grams.
Things you touch, wear, eat, and inhale have far higher levels of lead than those reported here.
Why do tampons have lead in them in the first place? Well, because they are made from cotton, which is a plant. Lead is an elemental metal that is ubiquitous on the planet. Plants in particular take up lead as they grow in soil - which means that plants will contain lead.
So let’s talk about some other plants you might encounter:
Textiles and clothing? Yep, also made with plant materials, including cotton.
The Consumer Product Safety Improvement Act (CPSIA) in the US has set a level of 90,000 ppb (90 parts per million (ppm)) for lead in accessible parts of children's products, including textiles. This was created specifically to help protect children from lead exposure, a population that is of particular risk for lead poisoning. That’s over 750 TIMES HIGHER than the mean lead levels detected in tampons.
The REACH (Registration, Evaluation, Authorisation, and Restriction of Chemicals) program by the European Chemicals Agency also sets limits on the use of lead in consumer products, including textiles. Their regulation is set even higher: 500,000 ppb of lead in textiles (including those with skin contact). That’s over 4,166 TIMES HIGHER than the mean lead levels detected in tampons.
Ok, I know what you’re going to say. Well, clothing, fabrics, furniture material, they’re only touching your external skin. The vaginal mucosa is different. A little bit, sure. But, the structure of the epithelium in your skin and the mucosa is actually pretty similar.
Let’s use some other mucosal membrane examples for the sake of argument: our gastrointestinal and respiratory tracts.
Tea leaves? Yes, plants you steep in hot water and then drink?
The European Union has a safety threshold of 5,000 parts per billion lead in food stuffs, including tea leaves. That’s over 42 TIMES HIGHER than the mean lead levels detected in tampons.
What about water? We ingest that and it comes into contact with lots of things that could contaminate it.
Tap water and bottled water are regulated by two different safety agencies. Tap water is regulated by the EPA, and bottled is regulated by the FDA. For tap water, there is a safety threshold of 15 ppb, and bottled water has a 5 ppb allowable level.
Say you drink 2 liters of water a day. For ease of math, 5 ppb also equals 5 micrograms per liter, and 15 ppb equals 15 micrograms per liter. That means, at these thresholds for water, something you ingest every single day, you could consume between 10 and 30 micrograms of lead daily.
10 micrograms equals 10,000 nanograms.
30 micrograms equals 30,000 nanograms.
To go back to our tampon example, let’s use the maximum detected level of lead: 936 nanograms in a 2 gram tampon. These levels in water you might consume daily are between 10 and 32 TIMES HIGHER than the lead detected in a tampon.
Alright, what about plants we burn and then inhale? Like… cannabis?
Cannabis is not federally regulated, so regulations vary by states that have legal weed growing practices.
California? The ultra-chemophobic state that, because of Prop 65, tells you *everything* causes cancer? Their limit on lead in cannabis is 500 nanograms per gram, which is high than the maximum lead concentration detected in a tampon in this study.
Washington state? 1,200 nanograms per gram.
Michigan? Even higher. 2,000 nanograms per gram in cannabis.
Colorado? The highest. 10,000 nanograms per gram in cannabis.
If you’re terrified of these news headlines regarding tampons, but not about these other things that contain far higher lead levels, you might want to explore your confirmation bias.
This study is the chemistry equivalent of using the wrong animal model.
So, these researchers decided to take apart tampons to assess what metalloids might be in the fibers. They used the inner absorbent core and the non-woven outer core.
These can be combinations of materials: cotton, rayon, polyester, polypropylene, and other additives like fragrance (depending on brand).
They used a method called Inductively Coupled Plasma Dynamic Cell Reaction Mass Spectrometry (ICP-DRC-MS) which can allow for analysis of trace levels of substances.
But if they wanted to assess trace levels that people would be realistically exposed to, they needed to process the samples using conditions that would simulate real-world conditions. They did not do that.
To extract substances from the tampons, they used 70% nitric acid combined with 350F heat.
I think everyone can agree, those are not the conditions inside a vagina.
What that means is that anything extracted from these tampons is not representative of what might leach out of a tampon during actual use of it.
For some comparison, a vagina is roughly 99F and a pH of 4.5.
Slightly acidic, but nowhere near the pH of 70% nitric acid, roughly 1.2. (pH units are orders of 10, which means that the nitric acid is nearly 2000 TIMES MORE ACIDIC than a vagina. Add to that the additional temperature, and this extraction doesn’t simulate anything that would occur in a biological model.
This means that we have no way of knowing whether these levels detected (assuming they are accurate, which we will get to), would even leach out of a tampon into a human body under physiological conditions.
What should they have done?
What I would have done - as a biomedical scientist - is try to mimic real-world conditions as much as possible. Soak a tampon in warm saline with pH adjusted to 4.5 and measure what leaches out into the solution. Honestly, this would have been less effort than what they did.
They could have been even more simple and just soaked the tampons in deionized water. That would have been even closer to the real-world conditions than what they actually did.
My guess? They tried those methods and nothing leached out of the tampons, so they needed to figure out a way to create a paper.
This is the problem with academic research. People receive grants to study a specific niche phenomenon, and then they are forced into a “publish or perish” cycle, where inconsequential and even unrealistic findings are submitted for publication into relatively weak journals.
And unfortunately, the research team on this paper doesn’t consist of anyone with biological science expertise.
The senior author is a geochemist, and the primary author is a postdoc in her lab with epidemiology and public health training. While the senior author understands chemical analytical methods, geochemistry expertise doesn’t mean you know how to conduct a biomedical study. I don’t see anyone on the co-author list that has expertise in biological sciences, reproductive anatomy, or physiology. Which is quite concerning based on the conclusions they’re making.
If they didn’t determine what would realistically leach out of tampons during real-world use, they certainly can’t make any statements related to potential absorption of those compounds.
This alone should is enough to toss out the findings as irrelevant.
This method is like saying they digested a fork using acid and saying there’s “toxic metals” in it that are being absorbed by your oral mucosa.
The study controls were not thorough enough to trust the results.
They use a reference material to help normalize their data, a cotton cellulose that contains 250 ppb lead (and other materials).
But what about other materials in tampons?
Why is there no reference for rayon, or polyester, or polypropylene? When doing analytical chemistry of MINISCULE levels of substances, you need to control for all variables that can skew the data and introduce bias in the results. Without other appropriate reference materials, they can't eliminate false positives or data point shifts as a function of the sample materials themselves.
What should they have done?
They should have had reference materials of the other polymers, and they SHOULD have used the digested sample materials (the tampons) as internal controls. This would involve taking the digested tampon materials and spiking them with known and measured amounts of all of the metals they wanted to test. This would have been a proper way to control the impact of the matrix and account for bias during mass spectrometry.
This is especially important when assessing lead and other materials that plant cellulose can pick up. Certain papers/filters cannot be used for blood analysis of metals because they routinely report false positives when you’re measuring trace levels such as is the case here.
Without these controls, you can’t conclude that the reported values are accurate.
The data have incredibly high variability even among their controls.
Another red flag is that they use this cotton reference material as a control: to say we are getting consistent data with this reference material per the certification documents it has, therefore we can trust our instrumentation and methods.
Unfortunately, their data don’t demonstrate that.
Looking at the intra-day and inter-day assessment of their reference material, they note variability in the values measured between 39% and 52%. That is incredibly high. For context, when I work with biological samples and replicates (which have more variability than chemical samples), we usually accept 10% or lower variability.
52% variability on their control? That wouldn’t be acceptable in any sort of regulated method.
This variability is very concerning, and can suggest issues with sample preparation, solubility of their substances and materials, issues with the instrumentation used to collect the data, and more. They did not address this.
But if their controls have this much variability, I am very skeptical that the findings they report are credible. And looking at their individual sample spread, you can see that there is just a phenomenal lack of consistency sample to sample.
When looking at data across brands (they lump samples from each brand here), there is just no pattern or trend to interpret. Could this be because their methods are flawed? Could this be because one manufacturer sources cotton from one part of the world with higher lead in the soil? Who knows. None of this was addressed.
This leads into the final point I’ll discuss.
The sample sizes are not representative enough to draw broad conclusions.
They tested 24 unique tampon samples. 24.
Those 24 tampons were inclusive of 14 different brands. They took 2 different samples from each tampon: not 2 different tampons, just technical replicates. For some reason, while they said they have 24 different tampon boxes, they ended up using (30) tampons. They don’t comment on why, but I wonder if it’s because of the variability in their data and trying to reconcile this to complete the story they’re creating.
Anyway, 30 tampons. Representing 14 brands. Of commercial products sitting on select shelves in various stores.
I think you can see where I’m going here. This is not a robust sample size. This does not represent the millions of tampons on the market, or the millions of tampons being used on a daily basis.
Now, while they do note this should be considered an exploratory study, that clearly was not emphasized in their comments in various news outlets, and certainly wasn’t noted by any of the clickbait headlines.
This is yet another reason why this study should have been nothing more than a footnote at least.
So why the fear-laden headlines?
There are a few things to blame. First, the public health departments of the schools that these researchers are affiliated with put out press releases.
Honestly, that’s reckless and irresponsible. A central tenet of public health is providing realistic information and nuance to people in order to improve public health. These press releases and subsequent media headlines do the opposite of that. They foment fear, erode trust in regulatory agencies that monitor consumer products, and erode science literacy.
Why? Likely to drive attention to their institution and increase the likelihood of funding. Another issue with the academic research infrastructure.
Media outlets don’t bother to actually review the study, but keep parroting the same talking points. They did not consult biomedical scientists to critically assess whether findings are relevant.
This team ignored relevant biology in their paper which is the biggest flaw of all.
The FDA has made a statement last night, which, is in line with what I just dissected here:
The FDA is reviewing the study. All studies have limitations. While the chemical method used indicates these metals are present in the tampons tested in the laboratory, the study does not assess whether any metals are released from tampons when used in the body. It also does not address whether any metal, if released, can be absorbed into the vaginal lining or, subsequently into the bloodstream. We plan to evaluate the study closely, and take any action warranted to safeguard the health of consumers who use these products."
Do you need to stop using tampons? No.
I will continue to use tampons (that’s my preferred menstrual product) because this study is really a nothing burger that has gone viral for no reason other than people like to profit off fear (yes, media outlets are making money every time you share their clickbait).
This study plays right into chemophobia, the irrational fear of chemicals. In a world where social media is plagued with outlandish claims causing fear while not focused on things that actually impact our health, scientists, and academic institutions, need to do better.
Detection does NOT equal relevance. This study has not demonstrated ANY relevance.
This is a classic case of much ado about nothing. Between the tiny sample sizes, methodological mishaps, and chemophobic fearmongering, there’s no solid evidence that the lead levels in tampons are a threat. The detected levels are below stringent safety thresholds, and there’s no reason to panic.
Please share this widely. If you are a journalist or affiliated with a media outlet that wants to do a better piece on this topic, please email me.
For more on related topics:
The word toxic is meaningless without context
The risk perception gap causes undue fear about things that pose little risk
Thanks for joining in the fight for science!
Thank you for supporting evidence-based science communication. With outbreaks of preventable diseases, refusal of evidence-based medical interventions, propagation of pseudoscience by prominent public “personalities”, it’s needed now more than ever.
Stay skeptical,
Andrea
“ImmunoLogic” is written by Dr. Andrea Love, PhD - immunologist and microbiologist. She works full-time in life sciences biotech and has had a lifelong passion for closing the science literacy gap and combating pseudoscience and health misinformation as far back as her childhood. This newsletter and her science communication on her social media pages are born from that passion. Feel free to follow on Instagram, Threads, Twitter, and Facebook, or support the newsletter by subscribing below: