Everything is chemicals—yes, even you
If a substance exists, it's made of chemicals. Sorry, not sorry!
This newsletter is free, but it’s able to sustain itself from support I receive from a small percentage of regular readers. If you value science-based information, consider upgrading to a paid subscription:
Welcome to the very first edition of Fundamental Elements, a new section of ImmunoLogic. My goal is to break down the basics of science fundamentals, so that you are better equipped to navigate the misinformation minefield that our society is living in. My hope is it will allow you to sniff out the clickbait, fearmongering, and pseudoscience, and ultimately make better informed decisions for you and your families.
To do that, we have to start at the beginning. That means we have to start with one of the most fundamentally (pun intended) misunderstood concepts: chemicals. And that’s because a lot of wellness misinformation exploits this misunderstanding. So if we master this concept, we are already making progress.
If you’ve ever heard someone say they “avoid chemicals,” you’ve met someone who doesn’t understand how reality works.
Because—spoiler alert—everything is chemicals.
Yes! Everything is chemicals. You’re a sack of chemicals. Your house is chemicals. Your pets are chemicals. Your food (all of it, not just the “toxic, artificial, ultraprocessed” stuff) is chemicals. Chemicals are the reason you, and everything else on the planet, exist.
Let’s get into it.
What even is a chemical?
I bet most of you have heard someone use the term “chemical” when they’re describing something as bad or harmful, right? Not going to inject those “chemicals” into my body—when referencing a vaccine. How can you drink that “chemical bomb” — when referring to a diet soda.
This is obviously not correct—but it is so entrenched because most people don’t realize that everything is chemicals. I know I use this phrase a lot, but it’s true. And repetition cements belief, right?
A chemical is anything that is made up of matter. That means if the substance has mass and takes up space—it’s a chemical.
The only things in our universe that aren’t chemicals are light and energy. So you literally cannot exist in a “chemical-free” world.
This includes:
Well, you. Your entire body, every organ, every tissue, every cell, every message sent between cells. All chemicals and networks of chemicals.
The air you breathe is a chemical mixture of various gases including nitrogen, oxygen, carbon dioxide. These are all chemicals.
Water you drink—H₂O. Yep, that’s also a chemical.
Caffeine in your coffee has the chemical formula C₈H₁₀N₄O₂. The coffee itself. All chemicals.
DNA (deoxyribonucleic acid) — those are the molecules that make up your genes. Those are chemicals too.
Proteins, fats, carbohydrates. Vitamins, minerals. All of them—chemicals.
Chemicals are substances. If a substance exists in a physical form—it’s chemicals.
Chemicals exist as elements and compounds
Elements are the building blocks of all substances—all chemicals.
Elements are pure substances—this means, they consist of only one type of unit of matter. That unit is the atom. That means elements are pure substances made up only of one type of atom.
Elements are what are listed on the periodic table—the foundation of chemistry.
Elements include hydrogen (H), oxygen (O), carbon (C), gold (Au), silver (Ag), Copper (Cu)…anything that’s listed on the periodic table.
Each element has specific and unique properties that defines their identity. Think of each element like a LEGO: they can be used to build more complex substances, both of repeated units of a single element, or in combinations of elements.
Compounds are made when elements get together
A compound is a chemical (or substance) that is made up of two or more different elements attached to each other. In chemistry, those attachments are called bonds.
The formation of that bond completely changes the properties of the new chemical when compared to the separate pieces (the elements).
This is so important to understand, because this misunderstanding is why people spread fear about “heavy metals” in vaccines, or tampons, or whatever the newest fear fad has become.
An example I’ve spoken about before: table salt.
Table salt is a chemical compound composed of sodium and chlorine.
Elemental sodium is a silver solid metal at room temperature. Sodium metal explodes when you put it into water. (This is real, I promise)
Elemental chlorine exists as a yellowish gas at room temperature, and can be extremely toxic with low exposures.
A chemical reaction causes the formation of sodium chloride, NaCl. The combining of these two elements makes a completely different chemical with completely different properties.
Sodium chloride is a white crystalline solid that dissolves in water that we use to impart flavor to foods (we call it table salt). The beauty of chemistry!
A chemical is NOT just the sum of its parts
Once these bonds form, the new chemical is completely distinct from the elemental “ingredients,” as I’m sure you gathered with the table salt example.
This is so important to understand. People often believe if an element is “toxic” on its own, then any compound containing it must also be dangerous. But that’s not how chemistry works.
Water (H₂O) is made from chemical reactions between:
Hydrogen (H₂) is an extremely flammable gas. (remember the Hindenburg?)
Oxygen (O₂) is also a gas that fuels combustion (fires need oxygen to burn)
Put them together and you get water, a liquid at room temperature which is essential for life, and can extinguish fires.
You don’t hear people saying water is filled with “toxic oxygen,” right? (pure oxygen is toxic at low doses, fyi) — or people saying water is filled with flammable hydrogen, either. That’s because people understand that water is a substance distinct from its parts, even if they don’t think about the parts.
Add another hydrogen to that water? You get hydrogen peroxide (H₂O₂)—which can be harmful if ingested, inhaled, injected, even topically or contact with the eyes.
Water and hydrogen peroxide are made up of the same elements, but they have different chemical formulas (and structures), which means: completely different effects.
So if someone says a chemical is “bad” because it contains a certain element, now you can teach them these chemical properties!
Chemistry is more than just mixing stuff together: it’s about how atoms interact
So we know that chemicals aren’t the sum of its parts, but how do those parts stick together? That’s the story of chemical bonding—you can think of it like molecular glue.
There are 3 main types of chemical bonds: covalent bonds, ionic bonds, and hydrogen bonds.
The type of chemical bonds in a chemical are determined by the elemental components and how those elements relate to each other. The type of bonds in a chemical gives us information about how reactive, soluble, stable, or useful a chemical might be.
Covalent bonds are bond where atoms share electrons to form molecules. Water is a covalent compound. Glucose is a covalent compound. DNA is a covalent compound.
Ionic bonds form when one atom gives away electrons and the other takes electrons. In this instance, each becomes an ion: one is positively charged when it is missing electrons and one is negatively charged with extra electrons. Those charged ions attract each other, allowing them to bond.
Table salt, sodium chloride, is an ionic compound formed with sodium ion (Na+) and chloride ion (Cl-). A fun fact? While we call table salt, salt, all ionic compounds are scientifically salts. Salts are ionic compounds: chemicals that are formed by positively charged and negatively charged ions. All those electrolytes in your energy drinks? Those are salts, aka ionic compounds.
A broad rule of thumb: if there is a metal element as part of the structure, the chemical often forms an ionic bond (this is not always true, though).
Hydrogen bonds are unique bonds that play important roles in biology that occur between hydrogen atoms (H) and certain other atoms like oxygen (O), nitrogen (N), and fluorine (F).
Chemical names aren’t “scary” — they tell scientists important information.
You’ve seen the claims, right? Don’t eat anything you can’t pronounce. If you don’t recognize a chemical name, then it’s gotta be harmful.
None of that is true, but it is a popular talking point among the wellness influencers like Vani Hari. In fact, the chemical name has literally nothing to do with whether a chemical poses any risk to us.
Chemical names tells us scientists important details about the chemical.
Many chemicals have common names and systematic names—often, the common names are what you might be familiar with, because it’s a lot easier to say table salt (or salt) than sodium chloride every time you grab for the shake, right?
There are even chemical identifiers which are used by scientists to classify chemicals and register them—this is especially important when we deal with chemicals with growing complexity and similar sounding names.
For accuracy and precision, we use a universal naming system from the International Union of Pure and Applied Chemistry (IUPAC). This ensures every chemical has a unique, standardized name, no matter where you are in the world. This gives us a language to speak to other scientists.
These names might sound complicated, even scary, but what they do is they tell us what atoms (or ions) are in the chemical, how many of them, how they are arranged, and how they behave.
Let’s illustrate with a couple examples:
Water is the common name for, well, water. Formula H₂O.
The IUPAC name for H₂O is… oxidane (while some people say dihydrogen monoxide, it’s not the systematic name). But scientists don’t even refer to water as oxidane, because water is so universal.
Vitamin C? Ascorbic acid? These are common names for the chemical with formula C₆H₈O₆,
But the systematic name for the exact same thing is (5R)-[(1S)-1,2-Dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one. Try to say that five times fast!
Baking soda, which you are familiar with when you make cakes or cookies (or maybe to deodorize your shoes), has chemical formula NaHCO₃. The systematic name for that is sodium bicarbonate.
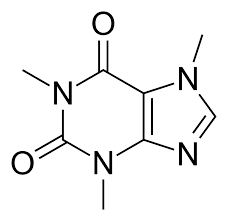
Caffeine? That delightful active ingredient in your morning coffee that helps with alertness? That’s also a common name for the chemical formula C₈H₁₀N₄O₂. The systematic name is 1,3,7-Trimethylpurine-2,6-dione.
Chemical names sound complicated because they follow those strict naming rules, but that doesn’t mean the substances themselves are dangerous. Think of it as a new language that scientists use to communicate important information.
Every chemical just is. No chemical is inherently “bad” or inherently “good” — they just exist (more on that soon).
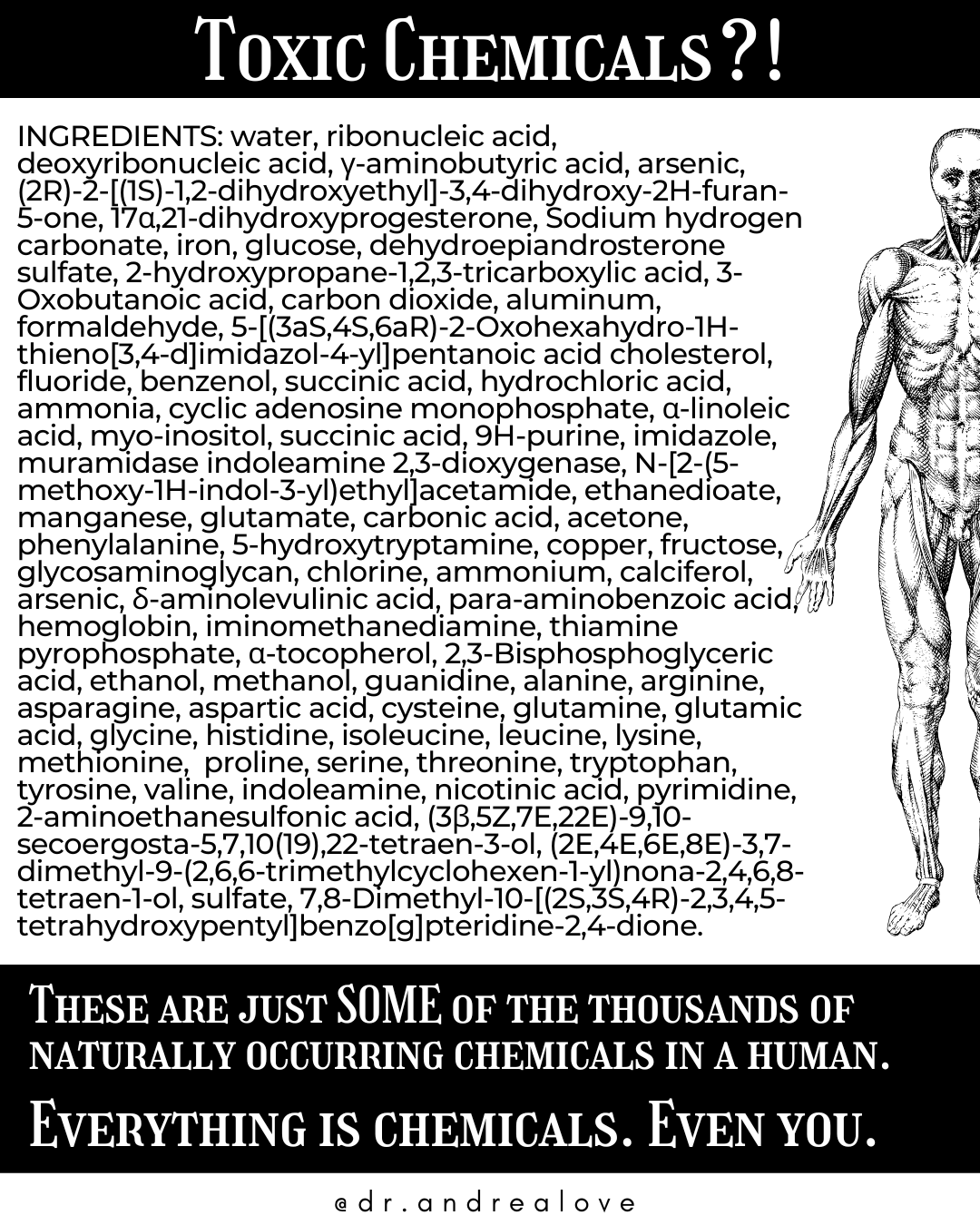
Some of the many many many chemicals you yourself is made up of:
Chemicals aren’t scary—they make up everything.
Chemistry is why life exists. Chemicals make up everything on this planet. Some might have simple names, some might have complex ones, some you might not be able to pronounce. Some just have complicated names because science requires precision.
Next time you see a fear-mongering post about “chemicals in food” or “chemical-free” products, remind yourself:
Everything is chemicals.
Long names don’t mean something is bad or harmful.
A compound isn’t the sum of its parts—it’s a completely different chemical than the starting elements.
It’s the full chemical formula and how those atoms are bonded that matter.
if someone is spreading messages like those, they’re doing it to scare you. Knowing these basics will help you see through the scaremongering. Don’t let those big words scare you: they’re just the language of the chemistry of the world.
Science isn’t scary, but misinformation makes it seem that way. We are going to fix that.
Now, more than ever, we all must join in the fight for science.
Thank you for supporting evidence-based science communication. With outbreaks of preventable diseases, refusal of evidence-based medical interventions, propagation of pseudoscience by prominent public “personalities”, it’s needed now more than ever.
More science education, less disinformation.
- Andrea
ImmunoLogic is written by Dr. Andrea Love, PhD - immunologist and microbiologist. She works full-time in life sciences biotech and has had a lifelong passion for closing the science literacy gap and combating pseudoscience and health misinformation as far back as her childhood. This newsletter and her science communication on her social media pages are born from that passion. Follow on Instagram, Threads, Twitter, and Facebook, or support the newsletter by subscribing below:









Some time ago, there was a kid whose science fair project was to see if he could get people to sign a petition to ban dihydrogen monoxide, claiming that it kills thousands of people each year due to accidental inhalation, is the major component of acid rain, contributes to the "greenhouse effect", could cause severe burns, contributes to the erosion of our natural landscape, accelerates corrosion of many metals, may cause electrical failures, causes decreased effectiveness of automobile brakes, and has been found in excised tumors of cancer patients.
As you may have guessed, his petition got a lot of signatures.
Thank you, Dr. Love! With the concerted attack on science (or even upon critical thinking…or thinking, period), we need you now, more than ever!