Every human has been exposed to this toxic chemical since birth
Spoiler: it's water. Welcome to Toxicology 101: where the dose, route, and exposure make the poison — not the name of the chemical.
This newsletter is free, but it’s able to sustain itself from support I receive from a small percentage of regular readers. If you value science-based information, consider upgrading to a paid subscription:
A quick reminder: Did you know that you don’t have to read my newsletters?
If you prefer listening in podcast-like format, the Substack app will read these articles aloud. Don’t let not having time to read stop you! Just open any article in the app and click the play icon.
We are back at it with the next edition of Fundamental Elements, a new section of ImmunoLogic. The goal? Break down science basics, address logical fallacies, and improve critical thinking, so that you are better equipped to navigate the misinformation minefield that our society is living in.
There is a logical progression in topics—think of it as following a course syllabus.
We can’t discuss more complex topics if we are at different places in our science understanding. If you missed the last one, I recommend you read it (below) first, as today’s article on the concept of toxicity, will build on that.
The timing couldn’t be better. I just returned from Vancouver, BC where I presented at the American College of Medical Toxicologists annual conference this past weekend.
My talk “Toxic Misinformation: How Chemical Fears Undermine Public Health” specifically discussed how this concept is manipulated by bad actors to scare people about things that don’t pose a risk to health — and how it harms all of us. I promise to write up a summary of it soon!
Cyanide is natural, Aspirin is synthetic. Which one do you trust?
The belief that “natural” is inherently better than “synthetic” is false — and it’s called the appeal to nature fallacy.
It convinces people that things (chemicals, substances, organisms) that are “natural” are better, good, beneficial, right, or safer simply because they are 'natural'. The opposite extends to anything “unnatural” — synthetic or artificial substances. People who fall prey to this believe that synthetic substances are inherently bad, harmful, dangerous, inferior, and unsafe.
You’ve heard it. You’ve maybe even said the phrase yourself:
“I don’t use [insert product here] — it’s toxic!”
The word toxic is a powerful word. It evokes fear, anxiety, and a sense of urgency. And why wouldn’t it? We all know, abstractly, that something that is actually toxic would cause harm, right?
The problem is, the word toxic is flung around like glitter, and almost always without essential context or understanding by the people using it. (parents, you get the glitter analogy, right?)
The word “toxic” is misused. A lot.
Let’s go back to basics.
What actually makes something toxic?
Spoiler: it isn’t what wellness influencers and media outlets claim.
Toxicology is the study of adverse effects of chemical, physical, or biological exposures on living organisms. Toxicology examines potential hazards, potential risks (the difference between a hazard and a risk is a big one, and we will revisit that too), understands how something may cause harm (the mechanism), and how people or regulatory agencies can reduce or avoid those potential risks.
From there, toxicity refers to a chemical, physical, or biological exposure that might cause harm to an organism, either directly or indirectly.
Scientifically, anything can be toxic at a certain exposure. Anything can be non-toxic at a certain [different] exposure.
Say it with me:
The dose makes the poison. With everything.
Toxicity is not about vibes. It’s about exposure — how much of a substance, how often, and how it enters your body. All of these elements are needed to determine if something is toxic:
The dose: how much of the exposure and for how long.
Dosages are usually normalized to exposure relative to body size (grams or milligrams or micrograms per kilogram of body weight)
The route of exposure: how are you interacting with the exposure
The specific chemical: remember, the source of a chemical does not matter — the actual chemical identity is what gives a substance it’s physical properties.
The species exposed: a human is not a plant, or an insect, or a mouse. Every organism interacts with chemicals differently.
That’s what matters. Because the same substance can be harmless in one scenario and harmful in another.
Science literacy tip: if someone claims something is toxic without all four of these elements, they are misleading you. Take that information with a very large grain of salt.
Toxicity is assessed by dosage per body weight.
Without that dosage, you cannot make any claims about whether something is “toxic” or “non-toxic,” because anything can be toxic at a high enough exposure.
This is usually represented by an exposure relative to the size of the organism (and species). Commonly, you’ll hear a value called LD50: this is a very important metric for toxicologists. This LD50 value is the 50% lethal dose: how much of a substance would kill half of a group of test subjects.
This value is typically assessed in toxicology safety studies in rodents (mice, rats) and other non-human mammals. It is also often converted into a human equivalent dose, especially for ingredients in medicines and foods, based on the chemical identity.
This is because animals and humans aren’t identical: it’s why onions and garlic are totally safe for you to ingest at high levels, but a low dose can cause kidney failure and death to your cat.
A higher LD50 value means the substance is less toxic.
That’s because that value is the dosage that can cause death, so a lower dose means a smaller amount can kill you. Keep that in mind as we discuss some examples.
Water is essential for life and…toxic.
Water (officially oxidane, if we use IUPAC naming) is essential for life.
Water makes up about 60% of our body. We must ingest water regularly in order to maintain our health and bodily functions.
But if you drink too much water too quickly? That water can turn from life-giving to toxic, quickly. That’s right: even water can be toxic. Drinking too much, specifically, more than 76 grams of water per kilogram of body weight, can kill you.
That’s roughly 5-6 liters (about 1.5 gallons) in ~3 hours for the average adult.
You can experience water intoxication, where excess water dilutes out critical chemicals (electrolytes, salts). Water intoxication causes nausea, vomiting, headache, confusion, seizures, coma and death.
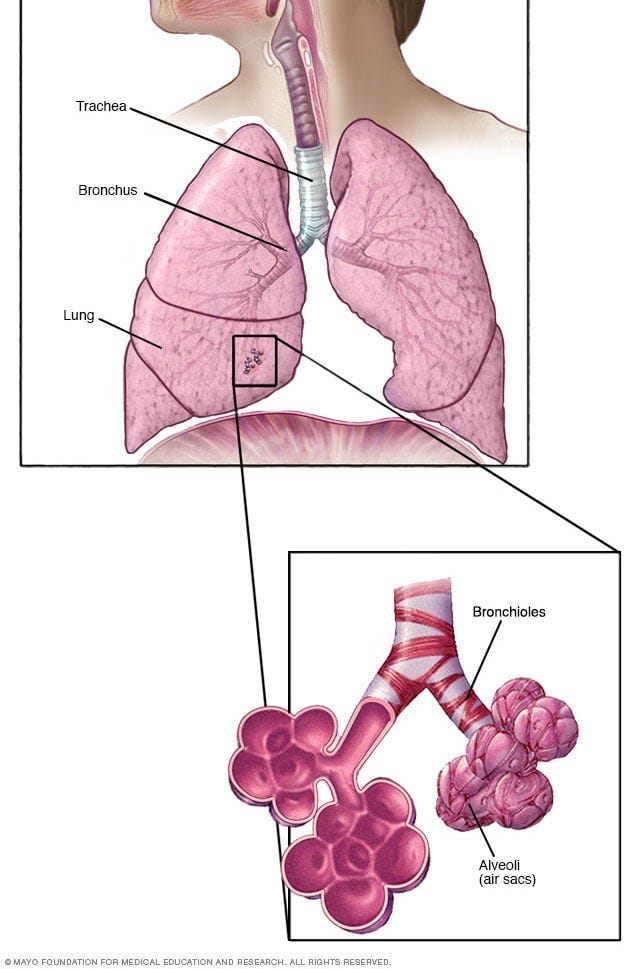
Now try inhaling water — that’s called drowning.
Just a few tablespoons aspirated into your lungs can be fatal. Water obstructs airways, prevent breathing, and causes suffocation. Water interferes with gas exchange in our lungs (how our lungs extract oxygen and excrete carbon dioxide). Water can prevent alveoli (the air sacs that do gas exchange) from functioning, causing low oxygen (hypoxia) and excess carbon dioxide (hypercapnia), both of which cause respiratory distress. Without medical intervention, this can lead to asphyxiation: brain damage, loss of consciousness, and death.
See how the dose and route of exposure matter?
Even substances or exposures essential for life or beneficial at a certain dose can become harmful [or toxic] at higher exposures or when you’re exposed through a different route.
Snake venom: deadly or digestible?
The Eastern Diamondback rattlesnake is one of the most venomous snakes in the US, but globally, doesn’t even break the top 10 (yes, I know, US has nothing on Australia).
If an Eastern diamondback bites and voluntarily injects venom (yep, snakes choose to inject venom, or not (a dry bite), venom will be injected intramuscularly, intravenously, or subcutaneously. When venom is injected, it can cause cellular death, tissue destruction, paralysis, and death — thanks to over 100 different chemicals.
Eastern diamondback venom is toxic at an injected dose of 1.2 milligrams per kg of body weight (that’s 84 milligrams total, if you’re a 70 kg (154 lb) person).
A bite can deliver up to 1,000 milligrams of venom, so that’s not great news for pretty much every human, considering less than 10% of that dose can be fatal. Mortality can be up to 30% among those with untreated bites.
Toxicity of venom is a result of over 100 chemicals (most of them proteins) with biological effects in our bodies. Eastern diamondback venom includes chemicals like:
Metalloproteinases: enzymes that degrade connective proteins like collagens, causing tissue damage, hemorrhage, and promote the spread of venom through the body.
Phospholipases: enzymes that degrade phospholipids, fats that make up membranes of our cells, causing cell death, tissue damage, and organ failure (since every part of our body is made up of cells).
Serine Proteinases: enzymes that degrade proteins involved in blood clotting. This is a double-edged sword: it can cause clots where you don’t want them (thrombus or embolus) but also causes bleeding where you don’t want it. The end result? Hemorrhaging, potential strokes, and death.
Myotoxins: proteins that degrade muscle cells, causing muscle failure, tissue damage, etc.
Neurotoxins: proteins that interfere with nerve signaling and nerve cell death, etc., causing paralysis, respiratory failure, and death.
and more.
Fun fact: the word toxin specifically means a chemical produced by a living organism that can be harmful at a certain dose. Think: botulinum toxin, tetanus toxin, myotoxins and neurotoxins (listed above). It doesn’t mean generic “synthetic” chemicals, which is the way wellness influencers frequently use it.
In fact, all true toxins are natural chemicals. Did I just blow your mind?
However, if you consume the same dose of snake venom, it’s unlikely to cause harm.
That’s because your stomach acid and proteinases (enzymes that degrade proteins) in your digestive tract would break down these chemicals and digest them into amino acid subunits like any other protein.
See the pattern? The dose and the route of exposure matter.
Your body is made up of chemicals that can be toxic at different doses.
Life, including human life, is a function of complex networks of chemicals. Proteins in your body? Made of chemicals. Every individual cell that fits together to create your organs, tissues, your entire body? All networks of chemicals.
Yes, your body is thousands of chemicals arranged in complex and intricate ways that make you who you are: a sack of chemicals. And yes, many are the same ones that are used to spread fear about foods, or vaccines, or consumer products in inflammatory and fear-laden posts across social media: formaldehyde, arsenic, alcohols, hydrochloric acid, ammonia, and more.
Say it with me: the dose makes the poison.
While you don’t want to exceed certain dosages of all of these chemicals, many of these are essential for life or are a function of metabolic processes in your body. And because the human body is way better at doing its job than wellness influencers would have you believe, it is really good at getting rid of stuff you don’t need.
Let’s take formaldehyde, since it is frequently used to scare people about vaccines.
There is naturally about 12,000 micrograms of formaldehyde in a pear. An infant produces about 1,100 micrograms daily. Adults? We produce up to 1.5 ounces (42,524 milligrams, 42,524,284 micrograms) of formaldehyde daily as a part of essential metabolic processes. Formaldehyde is found in trace levels in vaccines, as a result of some of the purification and inactivation processes during vaccine manufacturing (more here). Less than 0.1 milligrams would be found in any vaccine.
That’s 425,000 TIMES less formaldehyde than what an adult produces in their body on a DAILY BASIS.
So, do we need to fear formaldehyde outright? No. Even the levels in our bodies don’t pose a risk.
Nothing is automatically toxic OR non-toxic.
Non-toxic swaps you see all over social media? Those are marketing gimmicks or straight-up misinformation. Those “non toxic” swaps can absolutely be toxic - and many of them at lower doses compared to the alternatives those individuals are fear-mongering about. It’s easy to toss logic out the window when something sounds scary.
We have to consciously combat the tendency: remind yourself that everything is chemicals. Chemicals just are: they aren’t “bad” and they aren’t “good.” Remember the appeal to nature fallacy: the SOURCE of a chemical means nothing: whether a chemical is naturally-derived or entirely synthetic has no bearing on potential risk or safety of the chemical.
The actual chemical, the route of exposure, and the dosage of exposure are what matters.
Many chemicals have a specific mechanism or impact in one species, but not in another.
A common example is glyphosate, because it is used so frequently to scare people about food and their health. See here for a detailed piece on this chemical.
For example, glyphosate is frequently cited as being toxic, cancer-causing, and more. Glyphosate inhibits a key enzyme in plants required to make certain amino acids (plants photosynthesize, meaning they produce their own food, which is why they have this enzyme).
The enzyme glyphosate acts on does not exist in humans.
There is no physiological mechanism in which glyphosate would have that effect in people, even if we were exposed to a dose that could have that impact.
This is also why we interpret animal studies with caution. For example, when assessing food chemicals like aspartame, we factor in physiological differences between rodents, humans, and non-human primates.
It illustrates why media headlines claiming a chemical is “toxic” based on Petri dish studies cannot be taken at face value. It doesn’t mean the chemical has an impact in a realistic scenario. You can do ANYTHING to cells growing on a piece of plastic.
If you pour pure water onto cells in a Petri dish? They will explode. We know that’s not what happens to us when we drink water.
We can FURTHER refine our definition:
The dosage, the route of exposure, the chemical itself, and the mechanism in a certain species makes the poison.
Aspartame is demonized by wellness influencers as being toxic, yet is incredibly safe. It is 33 times less toxic than table salt, and 8.47 times less toxic than RFK Jr’s current supplement of the week, methylene blue.
Similarly, red dye 3 is claimed to be toxic, yet is 32 times less toxic than caffeine. And you’re exposed to far less red dye 3 than the caffeine most people willingly consume every day (and that dose of caffeine isn’t harmful, either).
Glyphosate is one of the safest herbicides in use, including compared to acetic acid, which is used as an herbicide in organic farming, and as your salad dressing. Glyphosate is nearly two-time less toxic than food-grade vinegar. And no one is actually ingesting meaningful levels of glyphosate (remember, those trace levels are in the parts per trillion: 1 gram per trillion grams of food).
If you hear someone saying some substance is TOXIC without context: they are misleading you, either intentionally or unintentionally.
Ask them: at which dosage? In which organism? If it is an insecticide or herbicide, does it have an impact in humans?
These questions help to tease out the reality from the sensational. Too often, sensational and unsubstantiated claims spread like wildlife on social media and through media outlets, but they do not reflect reality.
In this current infodemic of pseudoscience and anti-science rhetoric, we must all do our parts to fact-check and promote critical thinking. And that starts with basic chemistry and toxicology principles.
Science isn’t scary, but misinformation makes it seem that way. We are going to fix that.
Now, more than ever, we all must join in the fight for science.
Thank you for supporting evidence-based science communication. With outbreaks of preventable diseases, refusal of evidence-based medical interventions, propagation of pseudoscience by prominent public “personalities”, it’s needed now more than ever.
More science education, less disinformation.
- Andrea
ImmunoLogic is written by Dr. Andrea Love, PhD - immunologist and microbiologist. She works full-time in life sciences biotech and has had a lifelong passion for closing the science literacy gap and combating pseudoscience and health misinformation as far back as her childhood. This newsletter and her science communication on her social media pages are born from that passion. Follow on Instagram, Threads, Twitter, and Facebook, or support the newsletter by subscribing below: